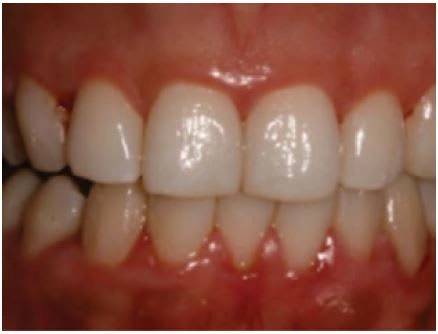
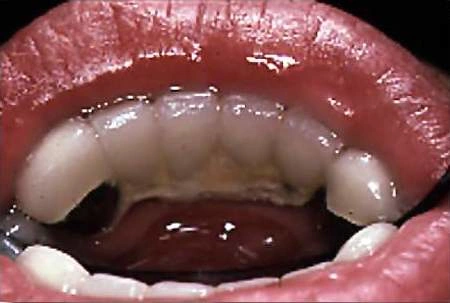
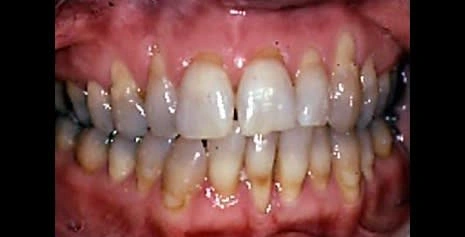
Gingivitis in a common oral disease, reported to affect 4 of 5 adults across the globe (Beaglehole 2009). The onset of gingivitis follows the accumulation of dental plaque and can be evident as early as 48 hours after dental plaque begins to form (Figure 3). Gingivitis can be prevented by maintaining low levels of plaque, and it can also be reversed (Tonetti et al. 2015).
Plaque produces an inflammatory reaction in the gingival tissues that results in increased blood flow and dilation of blood vessels. This is accompanied by an increase in all types of inflammatory cells, leading to swelling and reddening of the tissues after 48–96 hours. Continued exposure to plaque bacteria and their byproducts, such as metabolic toxins and proteolytic enzymes, promotes further inflammation and swelling, as well as engorgement and stasis of blood flow giving the tissues a bluish or purplish hue after fourteen to twenty- one days. At this point it is defined as an established gingivitis and it is not associated with irreversible damage. Without intervention, it may remain stable or progress to periodontitis with loss of attachment and destruction of the alveolar bone.
Figure 3. Gingivitis with redness and swelling
Figure 3. Gingivitis with redness and swelling
Gingivitis Research Summaries
Anti-Gingivitis Efficacy of a Stabilized 0.454% Stannous Fluoride Dentifrice: A Controlled 6-Month Clinical Trial
Assessment of the Effects of a 0.454% Stannous Fluoride Dentifrice on Gingivitis in a 2-Month Positive-Controlled Clinical Trial
A Randomized 2-Month Clinical Trial Evaluating the Anti-Gingivitis Efficacy of a Stabilized Stannous Fluoride Dentifrice versus a Triclosan Dentifrice
There are three ways in which gingivitis reductions can be achieved:
- Mechanical removal of plaque
- Anti-bacterial control of plaque
- Suppression of the host (human) inflammatory response
Gingivitis affects 4 of 5 adults globally
Gingivitis affects 4 of 5 adults globally
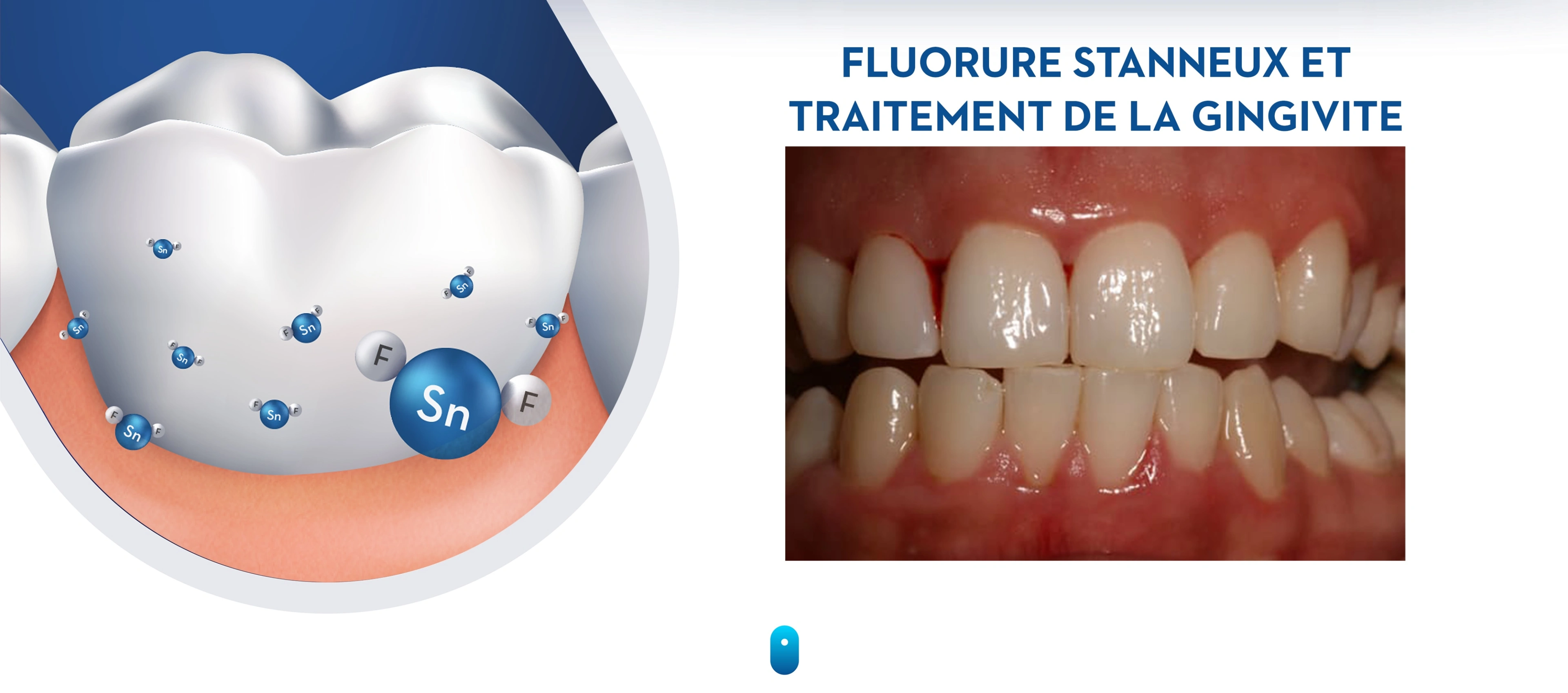
Mechanism of action of stannous fluoride
The reductions in gingivitis observed with stabilized stannous fluoride dentifrice are due to the broad-spectrum anti-bacterial activity of stannous fluoride (Ramji et al. 2005). Stannous fluoride inhibits bacterial metabolism, and thus reduces bacterial growth, bacterial adhesion and the production of toxins that potentiate gingival inflammation (Ramji et al. 2005, White 1995). Stannous fluoride also reduces the virulence of plaque by blocking the reactivity of LPS with tissue receptors that trigger inflammation (Haught et al. 2016a).
The stannous ion has high substantivity in the oral cavity, imparting a long-lasting antibacterial effect (Scott et al. 2009). Stannous levels in plaque remain above levels that are sufficient to inhibit metabolic activity up to twelve hours after exposure (Ramji et al. 2005). Otten et al. (2012) demonstrated that twelve hours after brushing with stabilized stannous fluoride dentifrice, plaque samples retained enough residual anti-bacterial activity to inhibit fresh, unexposed plaque samples. Given that dental plaque is associated with gingivitis, reducing and inhibiting plaque contributes to reductions in gingivitis. Retention of the stannous ion in plaque that remains after oral hygiene is important since the plaque that is missed during brushing is often in hard-to-reach areas where removal matters most to prevent the build-up of plaque and the onset of gingivitis.
Gingivitis Research Summaries
The following study summaries represent a sample of research demonstrating the benefits of stabilized stannous fluoride dentifrice for the reduction of gingivitis.
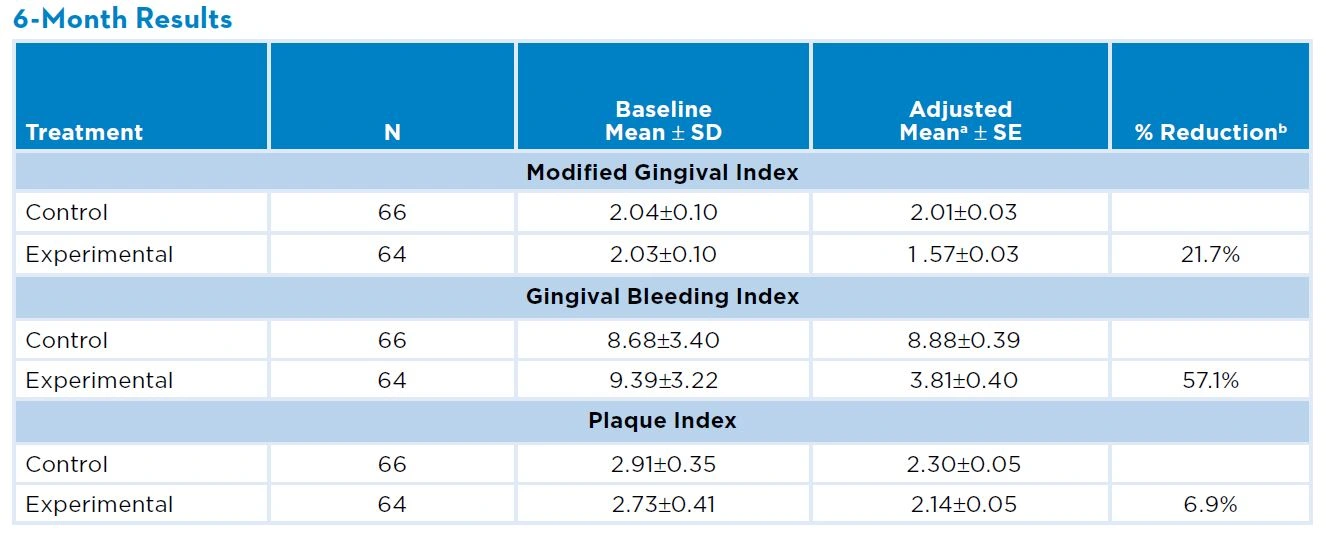
Reference: Mankodi S, Bartizek RD, Winston JL, et al. J Clin Periodontol. 2005;32(1):75-80.
Conclusion
Over a 6-month period a 0.454% stabilized stannous fluoride (Crest® PRO-HEALTH™) dentifrice showed a statistically significant and clinically relevant effect on the control and prevention of gingivitis compared to a negative control dentifrice (Colgate® Cavity Protection).
Objective
To compare the anti-gingivitis efficacy of a stabilized SnF2 dentifrice versus a positive control triclosan dentifrice over a 2-month period.
Methods
This was a randomized, positive-controlled, double-blind, parallel-group clinical trial involving generally healthy adults with mild to moderate gingivitis.
Qualifying subjects were randomized to one of two treatment groups:
- 0.454% stabilized SnF2 dentifrice (Crest® PRO-HEALTH™ Clean Mint [Smooth Formula], Procter & Gamble)
or
- Positive control dentifrice with 0.3% triclosan and 0.243% sodium fluoride (Colgate® Total®, Colgate-Palmolive)
Dentifrice was distributed over-labeled or over-tubed for blinding purposes, with a soft manual flat-trim toothbrush (Oral-B® Indicator™, Procter & Gamble). Subjects were instructed to brush with their respective dentifrice according to each manufacturer’s instructions.
The following efficacy and safety evaluations were conducted at Baseline and Month 2: Gingival Bleeding Index; Modified Gingival Index; and Oral Soft Tissue.
Treatment groups were compared using analysis of covariance with Baseline value as covariate. All statistical tests were two-sided with a 5% level of significance.
a Adjusted means and standard errors from analysis of covariance with baseline score as covariate.
b Percent reduction = 100% x (control-experimental mean)/control mean.
Anti-gingivitis Efficacy Of A Stabilized 0.454% Stannous Fluoride Dentifrice: A Controlled 6-month Clinical Trial
Key Clinical Results
Baseline values were balanced across the treatment groups (P>0.36) with overall baseline means of 2.09 for gingivitis, 15.8 for gingival bleeding and 15.6 for number of bleeding sites. Relative to baseline, both the stannous fluoride dentifrice group and the positive control group demonstrated a statistically significant (P<0.0001) reduction in gingivitis, gingival bleeding, and number of bleeding sites at Month 2.
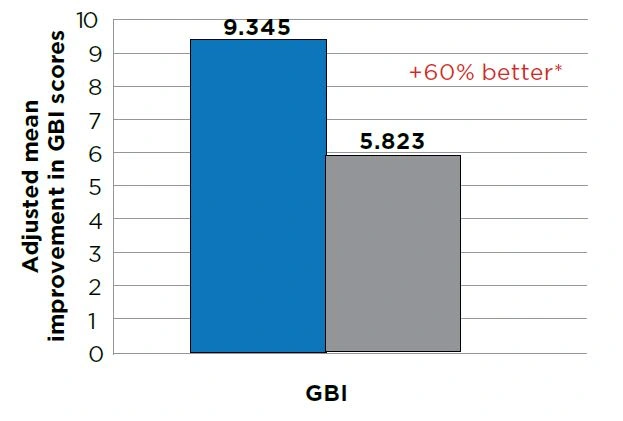
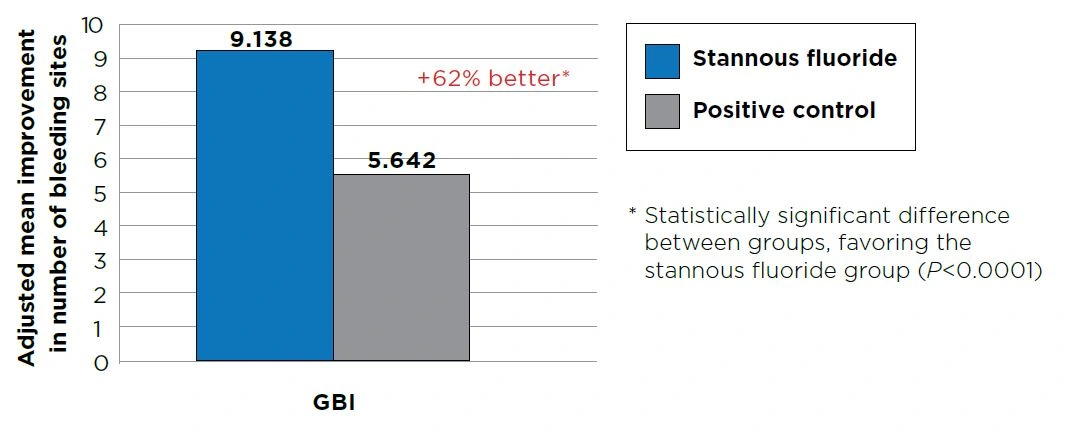
Between-treatment group comparisons for change from baseline showed the improvement from baseline for the stannous fluoride group was 45% greater for gingivitis, 60% greater for gingival bleeding and 62% greater for number of bleeding sites versus that of the positive control group (P<0.0001). See Figures 1–3.
At Month 2, the stannous fluoride dentifrice group demonstrated statistically significantly lower adjusted mean scores versus the positive control group for all 3 measures (P<0.0001).
Figure 1. Analysis of Covariance Summary for gingivitis (MGI). Improvement from baseline at Month 2.
Figure 1. Analysis of Covariance Summary for gingivitis (MGI). Improvement from baseline at Month 2.
Figure 2 - Analysis of Covariance Summary for gingival bleeding (GBI). Improvement from baseline at Month 2.
Figure 2 - Analysis of Covariance Summary for gingival bleeding (GBI). Improvement from baseline at Month 2.
Figure 3 - Analysis of Covariance Summary for number of bleeding sites. Improvement from baseline at Month 2.
Figure 3 - Analysis of Covariance Summary for number of bleeding sites. Improvement from baseline at Month 2.
Objective
To assess the effects of a 0.454% stannous fluoride dentifrice on the treatment of gingivitis as compared to a positive control dentifrice in a 2-month clinical trial.
Study Design
This was a randomized, positive-controlled, double-blind, parallel-group, single-center study with two treatment groups composed of healthy adult volunteers.
200 qualified subjects were enrolled; each treatment group contained 100 subjects. 99 subjects in the stannous fluoride group and 97 in the positive control group completed the study.
During the treatment phase, subjects performed their treatment routine unsupervised using their assigned dentifrice (Crest® PRO-HEALTH™ Clinical Gum Protection with 0.454% stannous fluoride, Procter & Gamble, or Colgate® Total with 0.3% triclosan and 0.32% sodium fluoride, Colgate-Palmolive) per manufacturers’ instructions (twice daily for stannous fluoride dentifrice; three times daily for the control) for 2 months. Both groups used an ADA soft reference manual toothbrush.
Efficacy measurements were obtained at Baseline and 2-months post-treatment. Antigingivitis efficacy was determined using mean Modified Gingival Index (MGI) and Gingival Bleeding Index (GBI).
Oral soft tissue and hard tissue assessments were conducted at each examination interval.
A Randomized 2-month Clinical Trial Evaluating The Anti-gingivitis Efficacy Of A Stabilized Stannous Fluoride Dentifrice Versus A Triclosan Dentifrice
Reference: CR Goyal1, JG Qaqish1, T He2, R Eusebio2. 1All Sum Research, Mississauga, Ontario, Canada. 2Procter & Gamble, Mason, OH USA
Key Clinical Findings
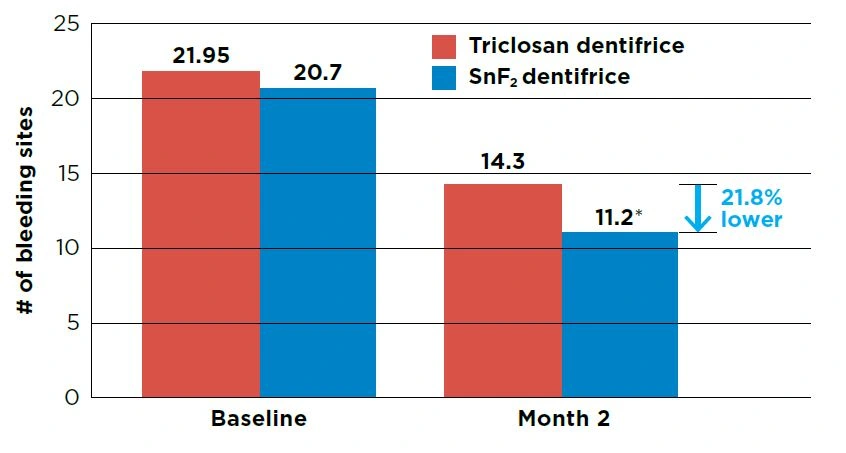
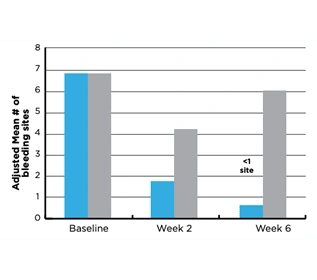
After 2 months of use, the stabilized stannous fluoride (SnF2) test dentifrice group had 21.8% fewer bleeding sites versus the triclosan positive control dentifrice group (P<0.001).
Both groups showed statistically significant reductions in bleeding sites from Baseline (P<0.0001).
Figure 1. Number of bleeding sites per group
Figure 1. Number of bleeding sites per group
- Significant difference between groups at Month 2, P<0.001. Groups were not significantly different at Baseline (P>0.05).
Objective
To compare the anti-gingivitis efficacy of a stabilized SnF2 dentifrice versus a positive control triclosan dentifrice over a 2-month period.
Methods
This was a randomized, positive-controlled, double-blind, parallel-group clinical trial involving generally healthy adults with mild to moderate gingivitis.
Qualifying subjects were randomized to one of two treatment groups:
- 0.454% stabilized SnF2 dentifrice (Crest® PRO-HEALTH™ Clean Mint [Smooth Formula], Procter & Gamble)
or
- Positive control dentifrice with 0.3% triclosan and 0.243% sodium fluoride (Colgate® Total®, Colgate-Palmolive)
Dentifrice was distributed over-labeled or over-tubed for blinding purposes, with a soft manual flat-trim toothbrush (Oral-B® Indicator™, Procter & Gamble). Subjects were instructed to brush with their respective dentifrice according to each manufacturer’s instructions.
The following efficacy and safety evaluations were conducted at Baseline and Month 2: Gingival Bleeding Index; Modified Gingival Index; and Oral Soft Tissue.
Treatment groups were compared using analysis of covariance with Baseline value as covariate. All statistical tests were two-sided with a 5% level of significance.
Clinical Comment
Gingival bleeding is an important early sign of gingivitis, the initial stage of periodontal disease. Reducing gingival bleeding is the ultimate goal of treating gingivitis, since research indicates the absence of gingival bleeding is a reliable indicator for sustained periodontal health.* This clinical trial showed subjects using the SnF2 dentifrice had significantly fewer (21.8%) bleeding sites than those using a positive control triclosan dentifrice after 2 months of use. Based on these findings, dental professionals should consider recommending the SnF2 dentifrice to patients with gingivitis to reduce bleeding and improve periodontal health.
* Lang NP. et al. J Clin Periodontol. 1990 Nov;17(10):714-21.
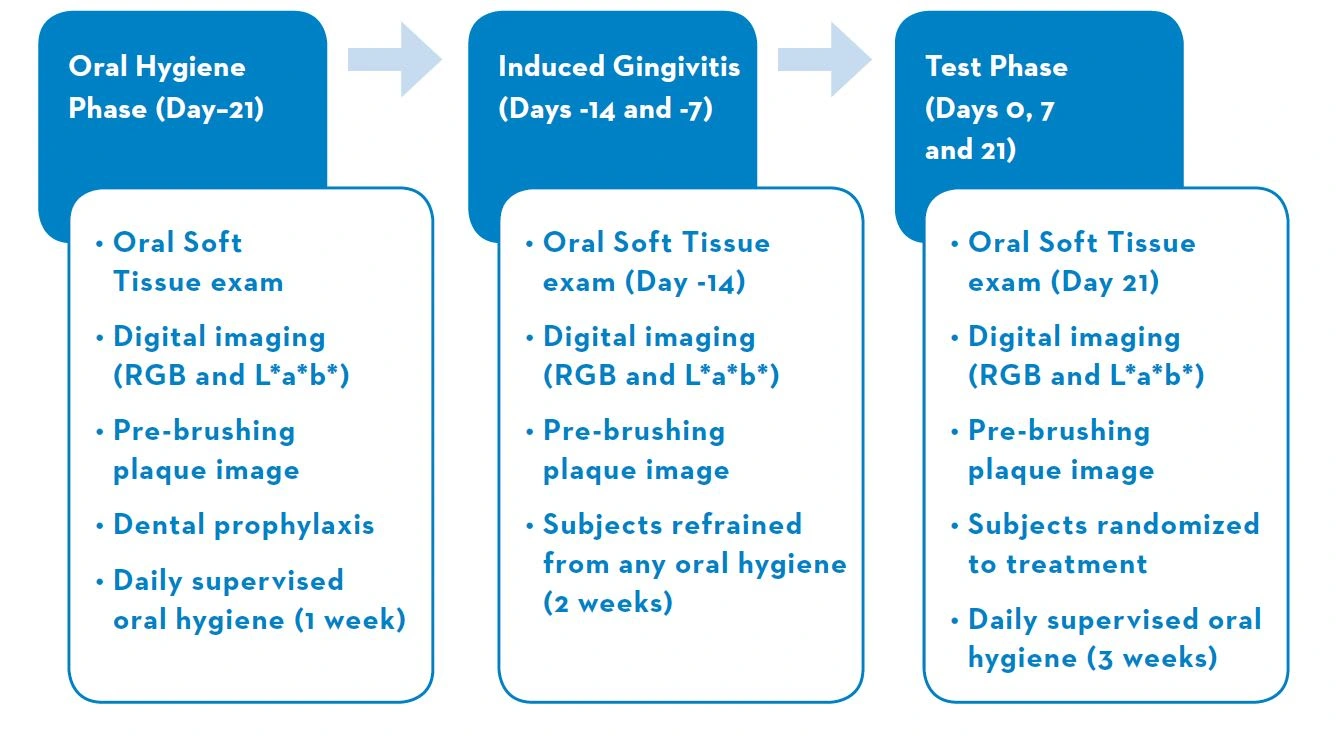
A Randomized Clinical Trial To Assess Gingivitis, Plaque, And Tooth Color After Use Of A Daily Two-step Dentifrice And Gel System Versus Chlorhexidine Rinse
Reference: Gerlach RW, Sagel PA, Barker ML, et al. J Dent Res 2015; 94 (Spec Iss A): Abstract 0293.
Key Clinical Findings
Overall
Plaque and Gingivitis
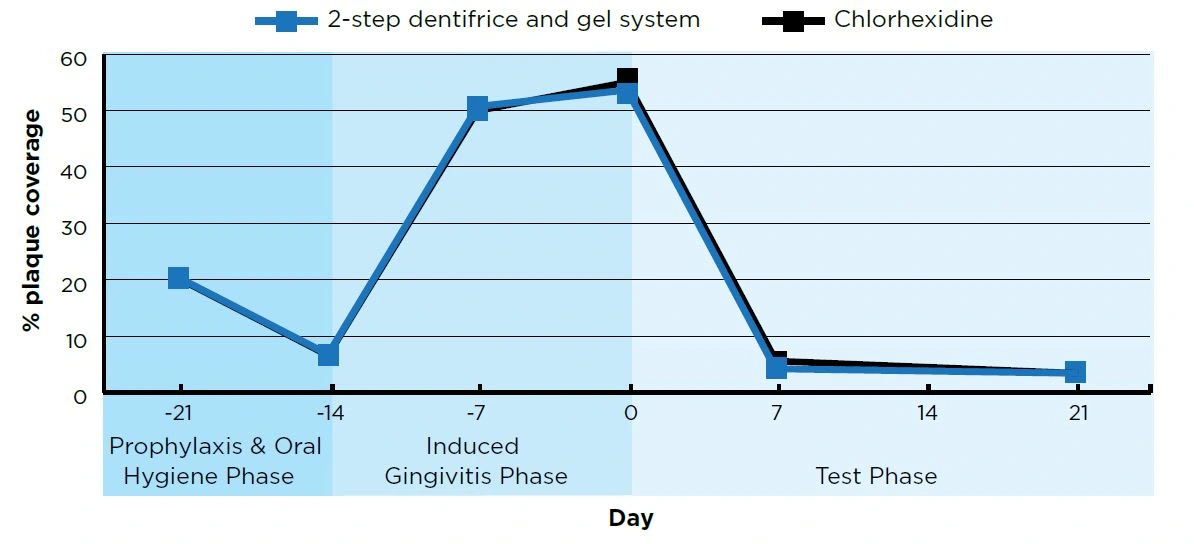
The daily 2-step dentifrice and gel system group and the chlorhexidine group had statistically significant (P<0.01) improvements in plaque area and gingivitis color measurements at both Day 7 and Day 21 from Day 0. See Figures 1 and 2.
There were no statistically significant differences between the 2-step dentifrice and gel system group and the chlorhexidine group in plaque and gingivitis reduction at Day 7 and Day 21.
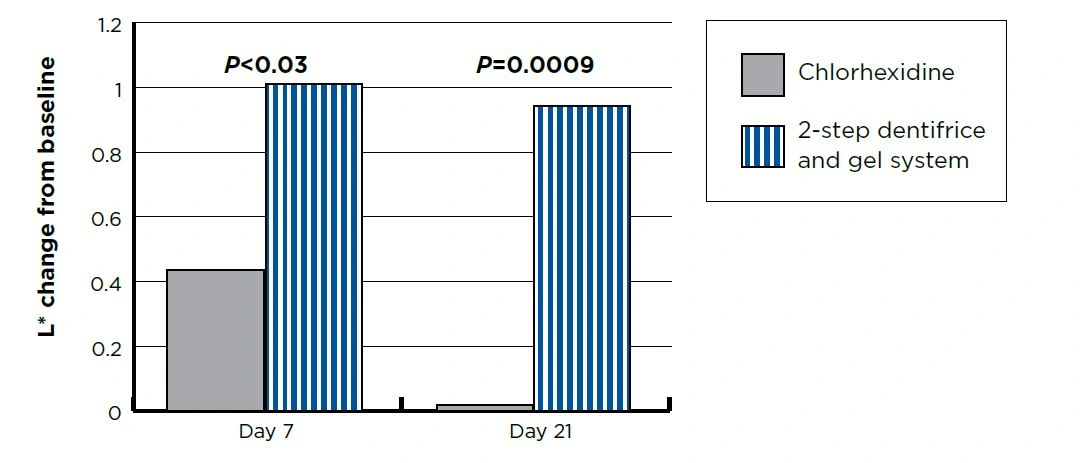
Tooth Color
Figure 1. Number of bleeding sites per group
Figure 1. Number of bleeding sites per group
- Day 7 and Day 21 are Means adjusted for Day 0. For both groups, Day 7 and Day 21 scores were statistically significantly different (P<0.0001) from Day 0.
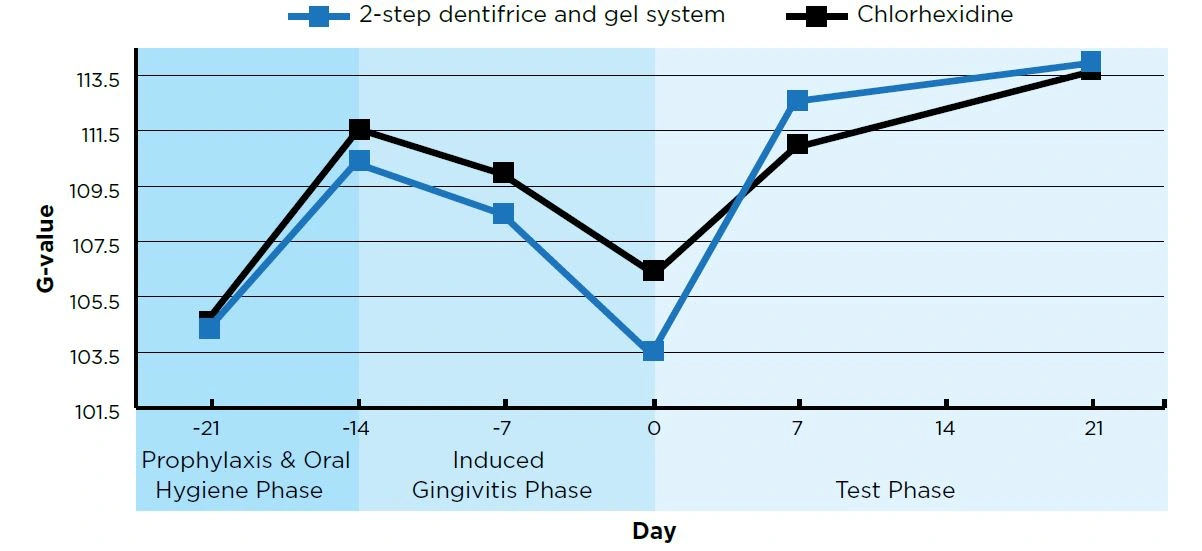
Figure 2. Gingivitis (Digital Gingival Imaging, a higher G-value indicates less gingivitis)
Figure 2. Gingivitis (Digital Gingival Imaging, a higher G-value indicates less gingivitis)
- Day 7 and Day 21 are Means adjusted for Day 0 For both groups, Day 7 and Day 21 scores were statistically significantly different (P<0.007) from Day 0.
Figure 3 - Analysis of Covariance Summary for number of bleeding sites. Improvement from baseline at Month 2.
Figure 3 - Analysis of Covariance Summary for number of bleeding sites. Improvement from baseline at Month 2.
Objective
To compare the anti-gingivitis efficacy of a stabilized SnF2 dentifrice versus a positive control triclosan dentifrice over a 2-month period.
Methods
This was a randomized, positive-controlled, double-blind, parallel-group clinical trial involving generally healthy adults with mild to moderate gingivitis.
Qualifying subjects were randomized to one of two treatment groups:
- 0.454% stabilized SnF2 dentifrice (Crest® PRO-HEALTH™ Clean Mint [Smooth Formula], Procter & Gamble)
or
- Positive control dentifrice with 0.3% triclosan and 0.243% sodium fluoride (Colgate® Total®, Colgate-Palmolive)
Dentifrice was distributed over-labeled or over-tubed for blinding purposes, with a soft manual flat-trim toothbrush (Oral-B® Indicator™, Procter & Gamble). Subjects were instructed to brush with their respective dentifrice according to each manufacturer’s instructions.
The following efficacy and safety evaluations were conducted at Baseline and Month 2: Gingival Bleeding Index; Modified Gingival Index; and Oral Soft Tissue.
Treatment groups were compared using analysis of covariance with Baseline value as covariate. All statistical tests were two-sided with a 5% level of significance.
Clinical Significance: Gingivitis
- These results demonstrate significant gingivitis and bleeding site reductions with use of stabilized stannous fluoride dentifrice.
- The comparative results demonstrate a significant improvement with stabilized stannous fluoride dentifrice compared to 0.3% triclosan/copolymer dentifrice in gingivitis and bleeding site reductions.
- These results indicate the significant reductions in gingivitis that can be anticipated with twice-daily use of 0.454% stabilized stannous fluoride-dentifrice as part of an oral hygiene regimen.