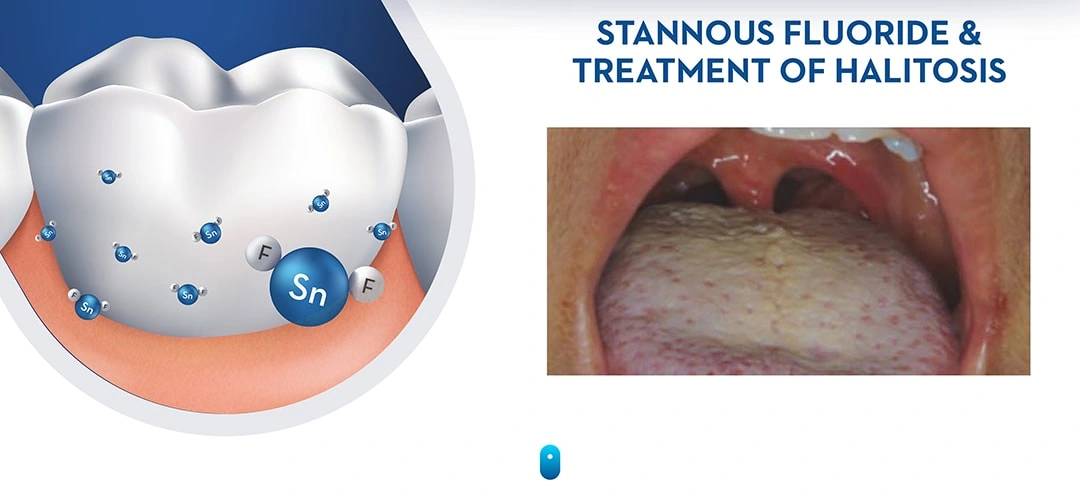
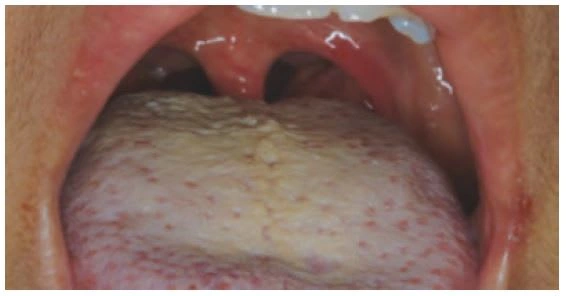
Halitosis is primarily the result of anaerobic Gram-negative proteins and producing volatile sulfur compounds (VSCs) – mostly methyl mercaptans and hydrogen sulphides (Tonzetich 1977). Oral malodor may also occur due to mouth breathing, oral infections, dietary constituents, as well as extra-oral factors. Meticulous oral hygiene reduces the level of oral bacteria, the production of VSCs, and therefore oral malodor. Tongue cleaning has also been recommended to help combat oral malodor since odorproducing bacteria commonly reside on the tongue (Figure 16; Outhouse et al. 2006; Tonzetich & Ng 1976, Van der Sleen et al. 2010).
Figure 16. Coating on tongue and heavy bacterial load
Halitosis Research Summaries
Effects of 0.454% SnF2 Dentifrice on Daytime and Overnight Malodor
Oral Malodor Reduction With 3-Week Use of 0.454% SnF2 Dentifrice
Stannous Fluoride Clinical Significance Oral Malador
Mechanism of action of stabilized stannous fluoride dentifrice
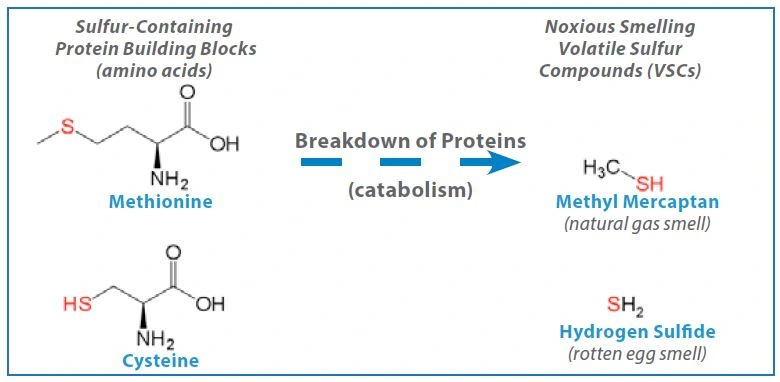
VSCs are the bacterial byproducts of metabolic activity, especially in anaerobic Gram-negative bacteria that proliferate on the tongue. Stannous fluoride exerts its anti-bacterial effect, primarily through metabolic inhibition. Ultimately, this leads to a reduction in the production of VSCs. Stannous ion can also bind directly to the sulfur sites in the sulfur-containing metabolic substrates (e.g. the sulfur-containing amino acids methionine and cysteine) creating competitive antagonism for their metabolism. The net effect of either mechanism of action is to reduce the level of foul-smelling VSCs (Figure 17)..
Figure 17. Source of Oral Malodor: GNA bacteria use protein as an energy source and produce volatile sulfur-containing by-products
Halitosis Research Summaries
Halitosis Research Summaries
The following study summaries represent a sample of research demonstrating the benefits of stabilized stannous fluoride dentifrice for reduction of breath malodor.
Effects Of 0.454% Snf2 Dentifrice On Daytime And Overnight Malodor
Effects Of 0.454% Snf2 Dentifrice On Daytime And Overnight Malodor
Reference: Farrell S, Gerlach RW, Barker ML, et al. J Dent Res. 2008;87(spec issue B): abstract 3161.
Conclusion
The use of the 0.454% stabilized stannous fluoride dentifrice resulted in significant reduction in short-term and long-term daytime and overnight malodor relative to a control dentifrice.
Objective
A clinical study was conducted to evaluate daytime and overnight oral malodor reduction benefit of a 0.454% stabilized stannous fluoride therapeutic dentifrice with short-term and long-term use.
Materials And Methods
The study was a randomized, double-blinded, 2-treatment, 3-period crossover clinical trial.
After completing an acclimation period, 45 subjects with existing oral malodor were randomly assigned to a crossover treatment sequence consisting of Crest® PRO-HEALTH™
dentifrice (0.454% stabilized stannous fluoride dentifrice) and Crest® Cavity Protection dentifrice (control).
For each treatment period, subjects brushed with the assigned product twice a day for 7 days. Oral malodor was assessed on a 9-point hedonic scale at baseline, day 2–overnight, day 2–daytime (4 hours post morning brushing), day 8–overnight, day 8–daytime (4 hours post morning brushing). Treatment periods were separated by washout periods during which subjects brushed with the control dentifrice.
Results
Subjects had a mean age of 39 years, 58% of the subjects were female and the mean baseline hedonic score was 7.4.
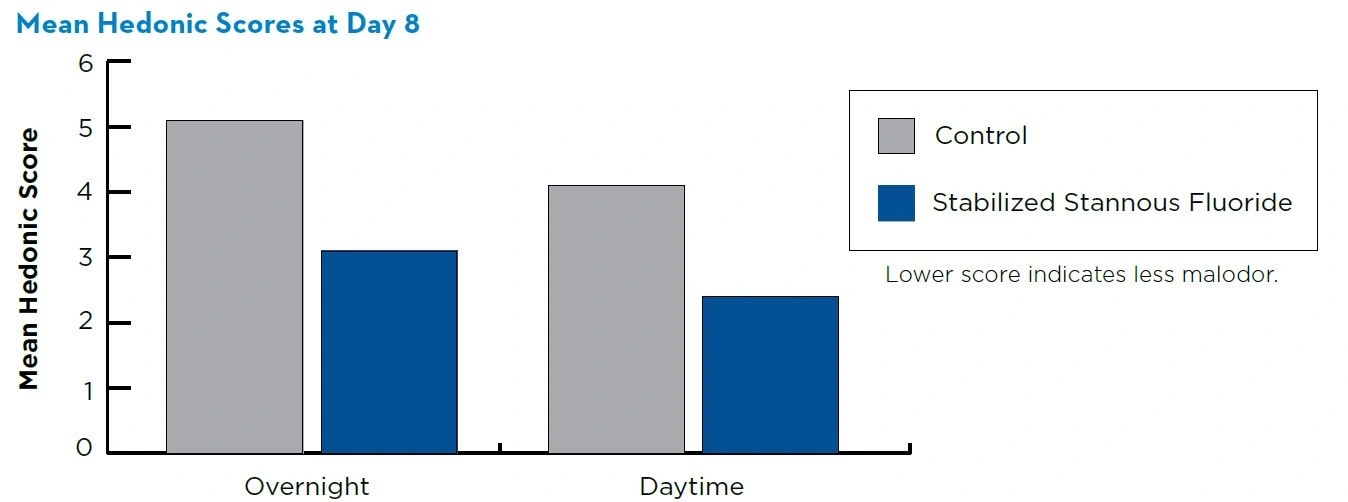
Relative to the control, use of the stabilized stannous fluoride dentifrice resulted in significant (P<0.002) improvement of the overnight and daytime malodor both short-term at day 2 and long-term at day 8.
The mean overnight hedonic scores were 3.2 and 5.1 at day 8 after 1 week of brushing for the stabilized stannous fluoride and the control dentifrices, respectively. The mean daytime hedonic scores were 2.4 and 4.1 at day 8 for the stabilized stannous fluoride and the control dentifrices, respectively.
Oral Malodor Reduction With 3-week Use Of 0.454% Snf2 Dentifrice
Oral Malodor Reduction With 3-week Use Of 0.454% Snf2 Dentifrice
Reference: Nachnani S, La S, Lee S, et al. J Dent Res. 2008;87(spec issue B):abstract 2864.
Conclusion
The use of the 0.454% stabilized stannous fluoride dentifrice resulted in significant reduction in short-term and long-term daytime and overnight malodor relative to a control dentifrice.
Objective
This clinical study evaluated the effects of the 3-week use of a 0.454% stabilized stannous fluoride therapeutic dentifrice on oral malodor.
Materials And Methods
The study was a randomized, double-blinded, 2-treatment, parallel design clinical trial.
After completing an acclimation period, 71 subjects with existing oral malodor were randomized to 1 of the 2 treatments: 0.454% stabilized stannous fluoride dentifrice (Crest® PRO-HEALTH™) or Crest® Cavity Protection dentifrice (control). Subjects brushed with the assigned product twice a day for 3 weeks.
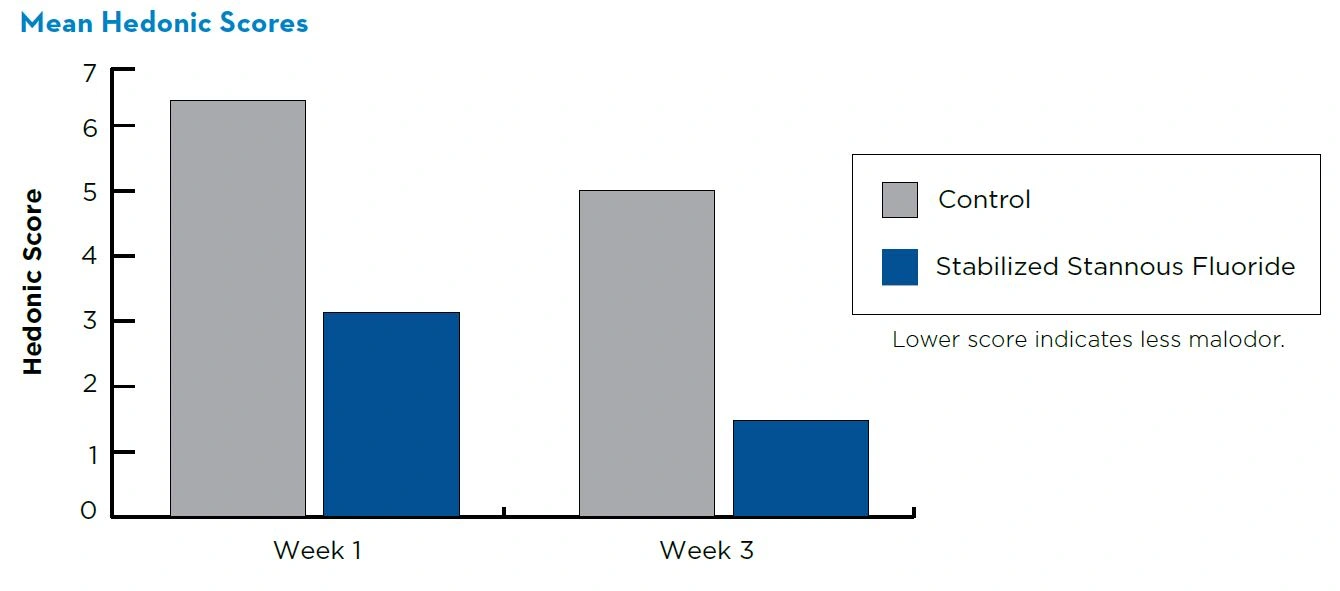
Oral malodor was assessed on a 9-point hedonic scale at baseline, week 1, and week 3.
Results
The mean age of study participants was 37.8 years, and 59% were female. The baseline mean hedonic score was 8.19.
At week 1, the mean hedonic scores (SE) were 3.40 (0.18) and 6.62 (0.18) for the stabilized stannous fluoride dentifrice and the control dentifrice, respectively.
At week 3, the mean hedonic scores (SE) were 1.55 (0.18) and 5.28 (0.18) for the stabilized stannous fluoride dentifrice and the control dentifrice, respectively.
Relative to the control, the use of the stabilized stannous fluoride dentifrice resulted in significantly (P<0.0001) greater reduction in oral malodor at both visits. Both treatments were well tolerated.
Clinical Significance: Oral Malador
Clinical Significance: Oral Malador
- Reducing oral malodor is a desirable patient benefit.
- Stabilized stannous fluoride dentifrice can provide the patient with short-term benefits, and long-lasting results, with twice daily usage.
- Multi-benefit stabilized stannous fluoride dentifrice offers the ability to control halitosis, along with many other important benefits.