The 2018 AAP/EFP Classification of Periodontal & Peri-implant Diseases
Course Number: 610
Course Contents
Key Dynamics Embracing Precision Medicine
The key dynamics driving the creation of the new periodontal disease classification encompassed findings from the Human Microbiome project and the concept of Precision Medicine. In 2007, the US National Institutes of Health (NIH) began a massive project that grew into an international initiative that is still underway. “The Human Microbiome Project.”13 These efforts are helping scientists and clinicians better understand how the human microbiome and their interactions with the human immune system either protect or harm the host. This information presents a more realistic view of the various microbiomes found in the human body and is helping clinicians understand how best to focus their efforts when treating their patients.
Findings from this project have revealed how important our microbiome is for our existence as humans and that microbes far outnumber human cells by more than ten-fold! The human microbiome is comprised of a “core” and a “variable” part.14 The core is shared among all humans, while the variable microbiome is exclusive to each individual based on their phenotype, genotype, and unique lifestyle.14 The differences in species and strains of this variable portion of the microbiome among individuals, may be as unique as their fingerprint! This also holds true for the oral microbiome.
Microbes do not typically occur in nature as a pure culture of a single species, but exist in a community of microbes, which are referred to collectively as a Microbiome. In the human host, the microbiome exists in several anatomical niches, each with their own exclusive microbiome and metagenome i.e., hair, skin, gastrointestinal tract, urogenital tract, vagina, nasal and paranasal sinuses, and the oral cavity. In ideal conditions, these microbiome niches represent species-balanced communities that have a symbiotic relationship in health. In the case of the oral microbiome, this symbiotic relationship plays a critical role in physiologic, metabolic, and immunological functions such as food digestion, energy generation and maintaining balance in pro-inflammatory and anti-inflammatory processes.15 This homeostasis is possible due to the presence of both commensal (beneficial) species as well as those capable of disease initiation (pathobionts). Although each of these microbial inhabitants residing within a unique ecosystem are geared towards symbiotic interactions within that particular ecosystem, if this balanced state is disrupted and disease-producing pathobionts begin to overpower the beneficial species, a state of dysbiosis ensues leading to intitial gingivitis. More recent theories have speculated that this imbalance occurs when the innate immune system by initiating the inflammatory response in its attempts to maintain homeostasis, fails when it is overpowered by certain keystone pathogens that further lead to this state of dysbiosis resulting ultimately in periodontitis.16 This new flipped hypothesis now questions whether it’s the microbes themselves or the inflammation that causes the dysbiosis.
The actual state of dysbiosis explains how oral diseases occur, more specifically this shift is most readily apparent in the microbial shift from gingivitis to periodontitis. The oral cavity houses the second largest number of microbiota next to the GI tract. Thus, the project has now developed organ-specific microbial databases for both the Human Intestinal Tract and eHomd (expanded Human Oral Microbiome Database). To date, almost 800 specific oral species have been added to the oral microbiome database but well over 1,000 are thought to exist.
This understanding has created a major paradigm shift in identifying the primary etiology of periodontal diseases. What is now considered to be old knowledge is that specific virulent periodontal pathogens cause periodontal tissue breakdown (mainly Gram-negative anaerobic bacteria). The problem with this theory is that no specific bacteria have ever been shown to be solely causative of periodontal disease. Thus, the new prevailing theory is that dependent upon the microbe-host environment, disease severity will vary. Periodontal breakdown in susceptible individuals creates an environment suitable for particular microbes, which then flourish. Certain keystone pathogens may be considered bridging organisms and may facilitate the transfer from symbiosis to dysbiosis in a susceptible host. Certain microbes may be found intraorally in health and maintain this healthy state, however interference with this symbiotic state leads to dysbiosis resulting in periodontal disease. It has also become clear with these new findings that rather than the old concept that all bacteria are bad, the main goal of oral hygiene should focus more on the maintenance of symbiosis rather than elimination of all oral microbes.
With this major shift in the understanding of the underlying causes of disease, an emerging approach for disease treatment and prevention that takes into account individual variability in genes, environment, and lifestyle for each person, has been created called “Precision Medicine,” previously known as personalized medicine. The major premise of precision medicine is a focus on identifying which treatment approaches will be the most effective for which patients based on genetic, environmental, and lifestyle factors. This is in contrast to the “one size fits all” approach that has dominated mainstream medicine and dentistry for decades.
Commercialization of the human microbiome as a drug therapy has already begun. In 2013, patients infected with C. dificile were successfully treated by duodenal infusion of the fecal microbiota of a healthy individual!8 The ability to use patients’ genetic and other molecular information as part of routine medical and perhaps dental care may soon be a reality. With these new discoveries in metagenomics and microbiomics, there will be improved ability to predict which treatments will work best for specific patients. Additionally, there will be better understanding of the underlying mechanisms by which various diseases occur leading to improved approaches to preventing, diagnosing, and treating a wide range of diseases.
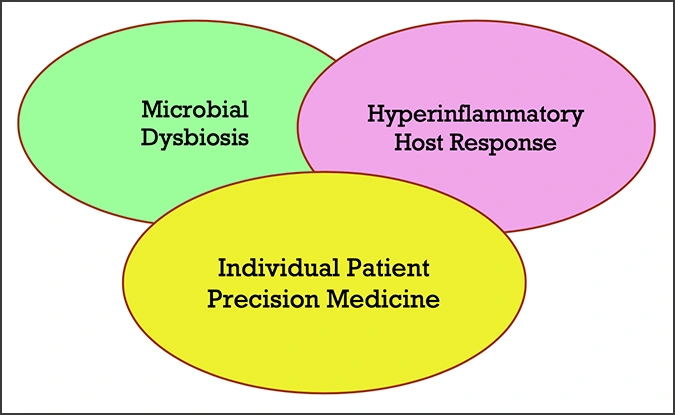
Because each individual harbors a unique microbiome that plays a key role in the etiology of disease within the body, disease may manifest and progress differently among different individuals, making precision medicine imperative for optimal health care. With this new knowledge, the focus of the new periodontal disease classification became one that is more biologically based, embracing the concept of Precision Medicine; one that encompasses both Microbial Dysbiosis and a Hyperinflammatory Host Response.
With this model in mind, the concept of creating more individualized patient case definitions came to fruition. Using the medical model of staging and grading, the authors of the new classification were able to combine some of the factors typically used in disease determination such as severity, extent, rate of progression, risk factors, etc. to create a method of more specifically defining individual cases. This system will be discussed in further depth under 5(b) Periodontitis.